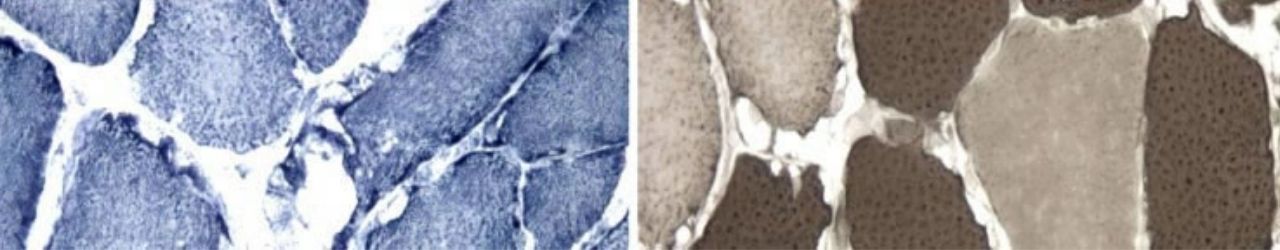
BIOCAPS Researchers will participate in a European Project on Neuromuscular Mcardle Disease
As well as McArdle Disease, EUROMAC also covers related muscle conditions that are even more rare.
 |
As well as McArdle Disease, EUROMAC also covers related muscle conditions that are even more rare. These include Glycogenosis Types 0, IV, VII, IX, X, XIII; Phosphoglycerate Kinase 1 Deficiency and Muscle Lactate Dehydrogenase Decifiency. EUROMAC arises from the international demand for cooperation on Rare Diseases, which led the European Commission and the US National Institute of Health to create in 2010 the International Rare Diseases Research Consortium (IRDiRC), with the mission of pursuing better diagnosis and treatment of rare diseases through the set up and maintenance of patient registries. Stemming from the experience gained in Spain by collecting data into a national registry of over 200 McArdle patients, EUROMAC addresses this mission and its expected impact by joining 15 partners from 7 EU countries, Turkey and the US. |
We have divided our work across 8 different working groups, dealing respectively with:
 |
Study of Ethical aspects related to the registry and set up of related procedures and forms, led by Assistance Publique Hospitaux de Paris; |
 |
Set up of the registry itself, led by the University Hospital 12 de Octubre, Madrid, based on their previous experience with the Spanish registry; |
 |
Analysis of the data gathered in the registry, led by Rigshospitalet University of Denmark; |
|
|
|
 |
Data and quality management, coordinated by the University of Larissa, Greece; |
|
|
|
 |
A thorough training and dissemination campaign aimed at spreading knowledge about McArdle Disease and other forms of rare muscular glycogenosis, led by the Association for Glycogen Storage Disease UK and University College London. Check out or news and events sections for future initiatives about McArdle targeted at both patients and health professionals; |
 |
Project Evaluation, lead by the Institute for Hospitalization and Scientific Care “Eugenio Medea”, Conegliano Veneto, Italy; |
 |
Project management and coordination, aimed at ensuring that EUROMAC runs smoothly as a whole throughout its 3 ½ years, carried out in Barcelona by the Vall D’Hebron Research Institute. |
WHAT IS THE REGISTRY OF PATIENTS AND WHY IS IT BEING CREATED?
EUROMAC registry is a secure, anonimised and confidential database designed to contain the medical data related to people with McArdle Disease and related conditions who give their consent to inclusion. The database will collate data from all people with the included conditions. EUROMAC aims to promote awareness and understanding of McArdle Disease and related conditions, to harmonise standards of diagnosis and care and to promote research.
EUROMAC hopes to recruit into the registry all those who are diagnosed with McArdle Disease and a number of even more rare related conditions. It will be seeking cooperation from medical professionals and patients to facilitate this. To be included, it is essential that the diagnosis is supported by DNA analysis. By being included in the registry, patients will be contributing to the well being of future generations of people with these conditions. Key aims being early diagnosis, high quality advice and management, and eventually an effective treatment or even a cure.
FUTURE STUDIES
The registry can be used in a number of ways but the key ones are:
for desk research
to identify suitable candidates for inclusion in future studies of treatments and trials of drugs.
EXPECTED LONG-TERM IMPACT
Improve access by patients to specialised care
Facilitate the involvement of national governments and regulatory agencies
Improve knowledge of the natural history of McArdle Disease and other rare neuromuscular glycogenoses
Reduce the average delay in diagnosis
Reduce risk of incorrect advice leading to debilitating symptoms and increased risk of life-threatening crises necessitating admission to critical care.
WHO IS RESPONSIBLE FOR THE REGISTRY? WHO SUPPORTS IT?
We are researchers in the field of neuromuscular disorders who investigate, at the clinical and research level, on glycogen storage disease type V (GSD V) (OMIM® number 232600), also known as McArdle Disease and other related ultra-rare muscle glycogenoses. We represent twenty institutes from across Europe, plus Turkey and the US, which make up the founding consortium of EUROMAC. The initial project started in March 2013, it will last just under four years and is coordinated by the Neuromuscular and Mitochondrial Pathology Group of the University Hospital Vall d’Hebron Research Institute in Barcelona, Spain.



























